1. Crush: Crush copper ore to particles:

2. Leaching: use dissolve sulfuric acid to leach out the metal ions. This can use heap leaching or pool leaching, should base on the content of copper.

3. Extraction: The leached ions should have Cu+, Fe+, Zn+, Mg+, Mn+, Ca+ etc. but our copper extraction reagent only extracting Cu+ out. The producing process is mixing and separation, now the copper ion is extracted to the organic phase (copper extraction reagent).


4. Reverse extraction: Use 180 g/L Sulfuric acid to reverse extraction copper ion out to get pure CuSO4.


5. Electrolyzation: Electrolysis the copper sulfate to get cathode copper:


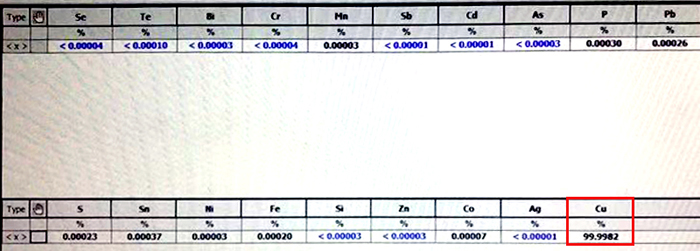
6. Our DZ988N’s selective of Cu:Fe is 2500:1, so the purity can over 99.96%. In practical, our engineer designed plant can get 99.9982% cathode copper.